
4 nuove sostanze SVHC dopo accordi nel comitato degli Stati membri.
Gennaio 9, 2017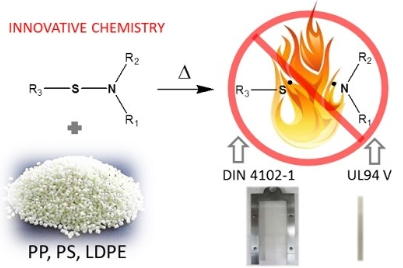
Azoto e zolfo per ritardare alla fiamma PP, LDPE e PS.
Gennaio 23, 2017The Member State Committee (MSC) unanimously agreed on the identification of four substances of very high concern (SVHCs): bisphenol A, PFDA, PTAP and 4-HPbl. The majority of the MSC’s members also supported the identification of an environmental endocrine disruptor PTBP and a respiratory sensitiser TMA as SVHCs.
Helsinki, 19 December 2016 – The Member State Committee (MSC) unanimously agreed on the SVHC identification of:
- 4,4′-isopropylidenediphenol (bisphenol A) (EC 201-245-8; CAS 80-05-7), proposed by France, due to its toxic for reproduction properties;
- nonadecafluorodecanoic acid (PFDA) and its sodium and ammonium salts (EC 206-400-3; CAS 335-76-2), proposed by Sweden, due to their toxic for reproduction and persistent, bioaccumulative and toxic (PBT) properties;
- 4-heptylphenol, branched and linear (4-HPbl) (EC -; CAS -), proposed by Austria, due to their endocrine-disrupting properties for the environment, and
- 4-tert-pentylphenol (PTAP) (EC 201-280-9; CAS 80-46-6), proposed by Germany, due to its endocrine-disrupting properties for the environment.
ECHA will include these substances in the Candidate List in January 2017.
For two other substances, the MSC acknowledged that there is scientific evidence that 4-tert-butylphenol (PTBP) (EC 202-679-0; CAS 98-54-4), also proposed by Germany, is an endocrine disruptor, and that benzene-1,2,4-tricarboxylic acid 1,2-anhydride (trimellitic anhydride, TMA) (EC 209-008-0; CAS 552-30-7), proposed by the Netherlands, is a respiratory sensitiser. For both, the majority of the members concluded, based on the overall assessment of all factors that the information provided is sufficient to constitute an equivalent level of concern to carcinogenic, mutagenic, toxic to reproduction (CMRs category 1A or 1B); persistent, bioaccumulative and toxic (PBT) and very persistent and very bioaccumulative (vPvB) substances. A few members, however, had different views.
As no unanimous agreement was reached by the MSC on the SVHC identification of PTBP and TMA, ECHA will submit the MSC opinions and the minority positions on these two substances to the European Commission. The Commission subsequently has three months to prepare draft proposals on their identification as SVHCs and take a final decision in its committee procedure.


